Innovation Summary
 It is argued that growing up in malaria endemic countries restricts early childhood development [1] preventing children from thriving and reaching their full social and economic potential in life.
It is argued that growing up in malaria endemic countries restricts early childhood development [1] preventing children from thriving and reaching their full social and economic potential in life.
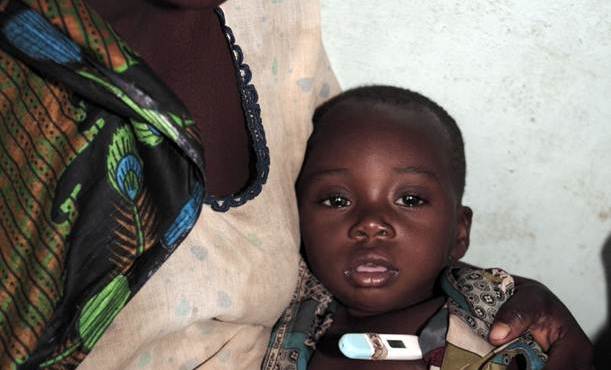
Malaria is not just a leading killer of children – it is also an important cause of morbidity. When malaria parasites infect of the central nervous system (CNS), symptoms such as seizures and coma can follow and children may be left with brain injury that is not immediately apparent but can cause a lifetime of disability. Brain infections can cause muscular paralysis, impairment in motor, visual or auditory function, memory, thinking, reasoning, speech and can provoke epilepsy. [2] Cerebral malaria affects 575,000 [3] children annually. Reliable data on risk and prevalence of later disabilities are scarce, and large scale evidence showing how malaria restricts human development potential is not available. Providing this evidence for policy change is the focus of the program.
Young children enrolled in a previous trial, who survived severe malaria, are being traced and assessed to determine whether and how the episode affected their brain. Reliable evidence on the nature and extent of compromised child development potential is being collected. This should spur efforts to prevent malaria and to increase access to anti-malarial drugs (including rectal artesunate) not just to avoid death, but to avoid future losses in child developmental potential.
Gallery
Impact
- ~12,000 episodes with symptoms of severe malaria were treated with a single dose of rectal artesunate or a placebo in the first intervention.
- Now, at least 7,500 children will be followed up from the previous study.
- ~3,000 children, some of whom had CNS malaria, are being assessed to determine the degree of disability associated with CNS infection on brain function and whether treatment modifies the effect.
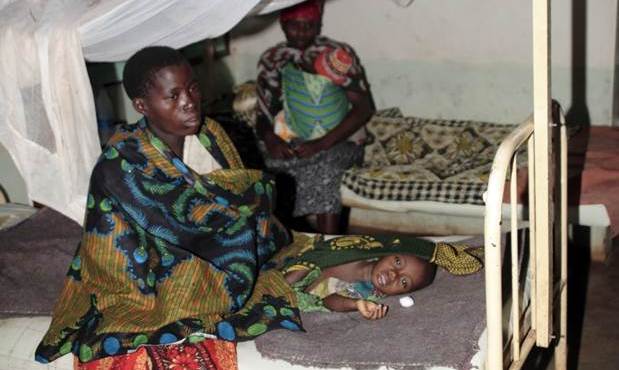
This child who is sick became ill when she was 3 years old. She had high fever and convulsions. She is now 11 years old. The fits she had then are still present today. She cannot talk, she cannot walk properly. She is doubly incontinent. She cannot ask for food. She moans a lot. She is completely dependent.
-- Rehema, mother of a young girl who had cerebral malaria
Innovation
The goal of the innovation is about early treatment of malaria so that lives are saved, and disability is prevented. It has been argued that the future economic development of malaria endemic countries could depend upon the elimination of malaria, because malaria restricts human development potential. Large scale evidence showing limitation of human development potential as a consequence of malaria is not available. Gathering evidence for policy change is our focus.
Everyone needs to understand what the consequences of delayed treatment are, in order to avoid harm. The way we are achieving this is to gather evidence that establishes the link between delayed treatment, CNS symptoms and short and long term disability so clearly that policy makers can act on the basis of the evidence.
Reliable evidence that early treatment of malaria – earlier than happens today – prevents death and disability is our focus. The ideal is for malaria to be avoided, or managed with oral medicines as soon as diagnosed. But if patients in the community have suspected severe malaria, can no longer be treated orally, and need immediate help, they should have emergency treatment with rectal artesunate and rapidly referred to the nearest hospital. Why? Because this prevents death, and lasting brain injury in the survivors.
For severe malaria, artesunate is now the best treatment. Given injectably, it saves more lives than quinine. [1, 2] Given rectally, before referral to hospital, a single dose reduces the risk of death or permanent disability in young children. [3]
Collaboration
Funders
- Grand Challenges Canada
Past Study Funders
- Tropical Disease Research Programme of the World Health Organization (Switzerland)
- UNITAID, Medicines for Malaria Venture (Switzerland)
- Orofino Pharma, (Italy)
- Catalent Pharma Solutions (Germany)
- Scanpharm (Denmark)
Key Partners
- Ministries of Health in Burkina Faso, Nigeria, Uganda, Malawi, Tanzania.
Implementation
Key Drivers
Reliable Follow-up, Ensuring Highest Standard of Implementation
The study is about reliable follow-up of all surviving children from a previous study on severe malaria. The purpose is to determine whether CNS malaria results in long term disability. The study is being implemented to the highest standards, and the highest quality of data is being obtained.
Challenges
Tracing and Re-Enrolling Children
Tracing and re-enrolling children from the earlier clinical trial was the main logistic challenge. Parents and patients had to travel a long distance to be assessed.
Continuation
Reliable evidence that early treatment of malaria prevents death and disability is the focus of the team working on this issue. The World Health Organization has already included the recommendation for early treatment with artesunate in its treatment guidelines. However, in order for individual countries to adopt global recommendations, they often need local data on the scale of the problem, and evidence on costs and feasibility of the intervention so that they know the policy implications.
A large study using the medication in Tanzania, Uganda, Ghana and Guineé Bissau has been completed. It is being submitted for publication. Further studies are about to be initiated in Nigeria, Uganda, Burkina Faso and Democratic Republic of Congo.
Evaluation Methods
All patients involved in the original trial were traced. Among the survivors, basic information was obtained on their vital, clinical and socioeconomic status. In addition, all patients who had CNS symptoms in the original trial plus a comparative sample of those who did not were assessed to determine whether CNS malaria increased risk for clinical consequences. Special attention was given to muscular paralysis, motor, visual, hearing, memory, reasoning, speech and epilepsy. More subtle consequences of malaria were also assessed including behavioural changes, cognitive development and educational, social and performance skills. Evaluation in the context of this study included all the parameters above.
Impact of Innovation
- The World Health Organization Malaria Treatment Guidelines now recommends emergency treatment with rectal artesunate as early as possible – i.e. as soon as malaria is suspected – if the patient is unlikely to be able to reach a hospital within 6 hours.
- The previous study found that if patients with severe malaria cannot be treated orally and access to injections will take several hours, a single inexpensive artesunate suppository at the time of referral substantially reduced the risk of death or permanent neurological damage. [3]
Cost of Implementation
Data from 2010 has shown that at low uptake and referral compliance (25%) of treatment, the intervention was estimated to avert 19 disability-adjusted life-years (DALYs; 95% CI 16–21) and to cost $1173 (95% CI 1050–1297) per DALY averted. Under the full uptake and compliance scenario (100%), the intervention could avert 967 DALYs (884–1050) at a cost of $77 (73–81) per DALY averted. [4]
The current study will provide more reliable estimates of DALYs.
References
- McCormick, M.C. et al. (1992). “The Health and Development Status of Very Low-Birth-Weight Children at School Age,” Journal of the American Medical Association, 267:16.
- Idro R. et al. (2010) Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatric Research, 2010 Oct;68(4):267-74.
- Murphy, S. C. et al. (2001) Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. The American Journal of Tropical Medicine and Hygiene, 2001 Jan-Feb;64(1-2 Suppl):57-67.
- Dondorp, A. et al. Artesunate Versus Quinine for Treatment of Severe Falciparum Malaria: a Randomised Trial. Lancet 2005, 366, 717-725.
- Dondorp, A. et al. Artesunate Versus Quinine in the Treatment of Severe Falciparum Malaria in African Children (AQUAMAT): an Open-Label, Randomised Trial. The Lancet 2010, 376, 1647-1657.
- Gomes et al (2009). Pre-referral rectal artesunate to prevent death and disability in severe malaria: a placebo-controlled trial. Lancet, 373: 557-566.
- Tozan, Y. et al. (2010) Prereferral Rectal Artesunate for Treatment of Severe Childhood Malaria: a Cost-Effective Analysis. Lancet 2010, 376, 1910-1915.
Resources
-
Research
-
van Hensbroek, et al. (1997) Residual Neurologic Sequelae After Childhood Cerebral Malaria. J Pediatr 1997, 131, 125-129.
-
Ngoungou, E. B., et al. (2006) Cerebral Malaria and Sequelar Epilepsy: First Matched Case-Control Study in Gabon. Epilepsia 2006, 47, 2147-2153.
-
Ngoungou, E. B., et al. (2006) Epilepsy As a Consequence of Cerebral Malaria in Area in Which Malaria Is Endemic in Mali, West Africa. Epilepsia 2006, 47, 873-879
-
Idro, R., et al. (2010) Cerebral Malaria: Mechanisms of Brain Injury and Strategies for Improved Neurocognitive Outcome. Pediatr Res 2010, 68, 1-2.
-
Idro, R., et al. (2006) Risk Factors for Persisting Neurological and Cognitive Impairments Following Cerebral Malaria. Arch Dis Child 2006, 91, 142-148.
-
Newton, C. R., et al. (1997) Intracranial Hypertension in Africans With Cerebral Malaria. Arch Dis Child 1997, 76, 219-226.
-
Holding, P. A., et al. (2001) Impact of Plasmodium Falciparum Malaria on Performance and Learning: Review of the Evidence. Am. J. Trop. Med. Hyg. 2001, 64, 68-75.
-
Holding, P. A., et al. (1999) Cognitive Sequelae of Severe Malaria With Impaired Consciousness. Trans R Soc Trop Med Hyg 1999, 93, 529-534.
-
Holding, P. A., et al. (2004) Assessing Cognitive Outcomes in a Rural African Population: Development of a Neurophychological Battery in Kilifi District, Kenya. J Int. Neuropsychol. Soc. 2004, 10, 246-260.
-
Carter, J. A., et al. (2004) Increased Prevalence of Epilepsy Associated With Severe Falciparum Malaria in Children. Epilepsia 2004, 45, 978-981.
-
Carter, J. A., et al. (2005) Persistent Neurocognitive Impairments Associated With Severe Falciparum Malaria in Kenyan Children. J. Neurol. Neurosurg. Psychiatry 2005, 76, 476-481.
-
Boivin, M. J. (2002) Effects of Early Cerebral Malaria on Cognitive Ability in Senalagese Children. J Dev. Behav Pediatr 2002, 23, 353-364
-
Boivin, M. J., et al. (2007) Cognitive Impairment After Cerebral Malaria in Children: a Prospective Study. Pediatrics 2007, 119, e360-e366.
-
Dugbartey, A. T., et al. (1998) Somatosensory Discrimination Deficits Following Pediatric Cerebral Malaria. Am. J. Trop. Med. Hyg. 1998, 59, 393-396.
-
Dugbartey, A. T., et al. (1997) Simpler Reaction and Cognitive Information Processing Efficiency After Cerebral Malaria in Ghananian Children. Neurol. Infect. Epidemiol 1997, 2, 141-144.
-
Carter, J. A., et al. (2006) Severe Falciparum Malaria and Acquired Childhood Language Disorder. Dev Med Child Neurol 2006, 48, 51-57.
-
Carter, J. A., et al. (2003) Neuro-Cognitive Impairment Following Acquired Central Nervous Infections in Childhood: a Systematic Review. Brain Research Reviews 2003, 43, 57-69.
-
John, C. C., et al. (2008) Cerebral Malaria in Children Is Associated With Long-Term Cognitive Impairment. Pediatrics 2008, 122, e92-e99.
-
Ngoungou, E. B., et al. (2006) Epilepsy As a Consequence of Cerebral Malaria in Area in Which Malaria Is Endemic in Mali, West Africa. Epilepsia 2006, 47, 873-879.
-
Carter, J. A., et al. (2003) Speech and Language Sequelae of Severe Malaria in Kenyan Children. Brain Injury 2003, 17, 217-224.
-
Holding, P. A., et al. (2004) Describing the Burden of Malaria on Child Development: What Should We Be Measuring and How Should We Be Measuring It? Am J Trop Med Hyg 2004, 71, 71-79.
-
Tozan, Y., et al. (2010) Prereferral Rectal Artesunate for Treatment of Severe Childhood Malaria: a Cost-Effective Analysis. Lancet 2010, 376, 1910-1915.
-
Mung'ala-Odera, V., et al. (2004) The Burden of the Neurocognitive Impairment Associated With Plasmodium Falciparum Malaria in Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2004, 71, 64-70.
-
Mung'ala-Odera, V., et al. (2006) Prevalence and Risk Factors of Neurological Disability and Impairment in Children Living in Rural Kenya. Int. J. Epid. 2006, 35, 683-688.
-
Biritwum, R. B., et al. (2001) Prevalence of Children With Disabilities in Central Region, Ghana. West Af. J. Med 2001, 20, 249-255.
-
Zaman, S., et al. (1990) Validity of the 'Ten Questions' for Screening Serious Chidhood Disability:Results From Urban Bangladesh. Int. J. Epid. 1990, 19, 613-620.
-
Holding, P. A., et al. (2009) Is Assessing Participation in Daily Activities a Suitable Approach for Measuring the Impact of Disease on Child Development in African Children? J Child & Adolescent Mental Health 2009, 21, 127-138.
-
Holding, P. A., et al. (2010) A Systematic Approach to Test and Questionnaire Adaptations in an African Context. 3CMC Proceedings 2010.
-
Khan, N. Z., et al. (2010) Validation of Rapid Neurodevelopmental Assessment Instrumente for Under-Two-Year-Old Children in Bangladesh. Pediatrics 2010, 125, e762.
-
UNICEF; University of Wisconsin. Monitoring child disability in developing countries. Results from the multiple indicator cluster surveys. 2008.
-
Durkin, M., et al. (1994) Validity of the Ten Questions Screened for Childhood Disability: Results From Population-Based Studies in Bangladesh, Jamaica and Pakistan. Epidemiology 1994, 5, 283-289.
-
Mung'ala-Odera, V., et al. (2004) Validity and Reliability of the 'Ten Questions' Questionnaire for Detecting Moderate to Severe Neurological Impairment in Children Aged 6-9 Years in Rural Kenya. Neuroepidemiology 2004, 23, 67-72.
-
Bradley, R. H., et al. (1996) Life at Home: Same Time, Different Places: An Examination of the HOME Inventory in Different Cultures. Early Dev. & Parenting 1996, 5, 251-269.
-
Chong, L. Y., et al. (2006) Is the Childhood Asthma Questionnaire a Good Measure of Health-Related Quality of Life of Asthmatic Children in Asia? Validation Among Paediatric Patients With Asthma in Singapore. PharmacoEconomics 2006, 24, 609-621.
-
World Health Organization. International Classification of Functioning, Disability and Health. 2001.
-
The Weschsler Intelligence Scale for Children; The Psychological Corporation; New York: 1949.
-
Kaufman, A. S., et al. (2004) Assessment Battery for Children; Circle Pines, MN:American Guidance Service.
-
Gioia, G. A., et al. (2000) Behavior Rating Inventory of Executive Function. Psychological Assessment Resources.
-
Banu, S. H., et al. (1993) Profile of Childhood Epilepsy in Bangladesh. Developmental Medicine & Child Neurology 1993, 45, 477-482.
-
Fosgren, L. (2008) Estimations of the Prevalence of Epilepsy in Sub-Saharan Africa. Lancet Neurology, 7, 21-22.
-
-
Instruments & Batteries
-
Clinical: visual assessment, audiometry, history of convulsions, acute episodes (CNS and non-CNS), hospitalization, epileptic seizures, treatment, diagnosis, electroencephlography (EEG), Magnetic Resonance Imaging (MRI)
-
Psychology: Atlantis – General Intelligence; Hand Movements General Intelligence; FootSteps - General Intelligence; Story Completion - General Intelligence; Kilifi Naming Test - General Intelligence; Observational - General Intelligence; Language Checklist - General Intelligence; Shift - Executive Function; No Go - Executive Function; People Search - Executive Function; Reading Age & Computational Age - Literacy; BRIEF - Socioemotional
-